Background
Prospective randomized trials have reported a benefit for ATG-based GVHD prophylaxis in the setting of allogeneic hematopoietic stem cell transplantation (alloHSCT) with an unrelated donor (UD). However, the best GVHD prophylaxis has been recently challenged by the use of PTCY, first in haplo-identical transplant and then in HLA mismatched or matched unrelated transplantation. We here present the outcomes of PTCY vs ATG as GVHD prophylaxis in MDS patients from the EBMT registry transplanted with an unrelated donor.
Method
A total of 969 patients transplanted from 2012 to 2019 in 92 participating centers, receiving either PTCY or ATG, were included in this study. The primary outcomes were progression free survival (PFS) and overall survival (OS). Competing risks analyses were performed to analyze non-relapse mortality (NRM) with competing event relapse, and acute GvHD grade II-IV, chronic GvHD and neutrophil engraftment with competing events relapse and death. Cox proportional hazards regression with a predefined covariate constellation (age, IPSS-R cytogenetics, donor HLA matching, age, hypomethylating agents (HMA) prior to transplantation, comorbidity score and MDS classification (MDS with or without excess blast, EB) was used to obtain adjusted effect estimates of ATG compared to PTCY for OS and PFS.
Results
Overall, 214 patients received PTCY and 755 received ATG as GVHD prophylaxis for UD alloHSCT. Patients of the ATG group were older (60.1 year-old [IQR: 50.6-65.2] vs 57.2 year-old [IQR: 46.4-61.1], p=0.008). Disease characteristics were similar in both group: there were a total of 583 MDS with EB at diagnosis, 510 MDS-EB at transplantation, and 207 patients transformed into AML. Among patients with available data for IPSS-R at MDS diagnosis (n=760) 44 (5.8%) were Very Low, 114 (15%) Low, 209 (27.5%) Intermediate, 232 (30.5%) High and 161 (21.2%) Very High. Patients of the PTCY group received more frequently HMA before transplantation (72.4% vs 57.3%, p=0.001) and were preferentially transplanted from an HLA mismatched 9/10 donor (37.9% vs 21.6%, p<0.001). Conditioning regimen was more frequently myeloablative in the PTCY group (55.4% vs 38.7%, p<0.001). Neutrophil engraftment at 28 days was significantly better with ATG (93% vs 84%, p<0.001). With a median follow-up of 4.4 (95% CI 4.2 - 4.8) years, five-year OS was 57% (49-64) with PCTY and 49% (95% 46-53%) in ATG group, p=0.127. Five-year cumulative incidence of relapse was similar in both groups (22%, 95%CI 16-29 in PTCY vs 25%, 95%CI 22-28 in ATG; p=0.28). Notably, 5-year PFS was marginally higher for PTCY with 52% (95%CI 44-59) vs 44% (95%CI 40-48) for ATG, p=0.075, whereas 5-year NRM was 26% (95% CI 20-32%) vs 31% (95% CI 27-34) in PTCY and ATG, respectively, p=0.28. Grade II-IV acute GVHD incidence was lower using PTCY (22% [95% CI 17-28%] vs 30% [95% CI 26-33%]), p=0.035 while there was no difference for chronic GVHD (36% (29-43% with PTCY vs 38% (34-41%) in ATG at 5 years).
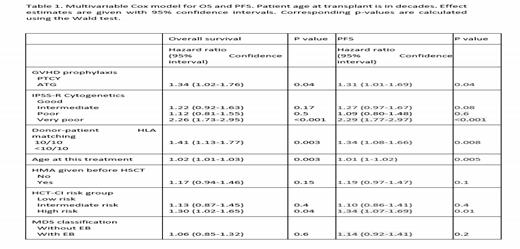
Multivariable analyses confirmed a better OS and PFS for PTCY, with a HR of ATG of 1.34 (95% CI 1.02-1.76), p=0.04, and a better PFS for PTCY with a HR for ATG of 1.31 (95%CI 1.01-1.69) (Table 1). There was no apparent interaction between GvHD prophylaxis and IPSS-R or HLA matching, suggesting an effect exclusive to the type of GVHD prophylaxis used.
Conclusion:
This EBMT registry study suggests that MDS patients who received a transplant from an unrelated donor had a better OS and PFS using PTCY compared to ATG. This confirms that PTCY instead of ATG in this setting is a valid option.
Disclosures
Chalandon:MSD: Honoraria, Other: travel support; Novartis: Honoraria, Other: travel support; Astra-Zeneca: Honoraria, Other: travel support; Sanofi: Other: travel support; Servier: Honoraria; Gilead: Honoraria, Other: travel support; Jazz: Honoraria, Other: travel support, Speakers Bureau; Roche: Honoraria, Other: travel support; Abbvie: Honoraria, Other: travel support; Pfizer: Honoraria; Janssen: Other: travel support; Incyte: Honoraria, Other: travel support; BMS: Honoraria, Other: travel support; Amgen: Honoraria, Other: travel support. Ciceri:ExCellThera: Other: Scientific Advisory Board . Kröger:MSD: Honoraria; Neovii Biotech: Honoraria, Research Funding; BMS: Honoraria, Research Funding; Pfizer: Honoraria; Riemser: Honoraria, Research Funding; Novartis: Honoraria, Research Funding; Takeda: Consultancy; Jazz: Honoraria; Kite/Gilead: Honoraria; Sanofi: Honoraria. Yakoub-Agha:Janssen: Honoraria; Bristol-Myers Squibb: Honoraria; Novartis: Consultancy, Honoraria; Kite, a Gilead Company: Consultancy, Honoraria, Other: Travel Support. Rambaldi:Abbvie: Honoraria. Scheid:Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; BMS: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Amgen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Abbvie: Honoraria; Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Roche: Consultancy; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees. McLornan:UK ALL RIC TRIAL - DSM board: Other: participation on a data safety monitoring board or advisory board; EBMT Scientific Council Member: Other: Chair of EBMT CMWP; Jazz Pharma: Honoraria; Abbvie: Honoraria; Novartis: Honoraria; Imago Biosciences: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal